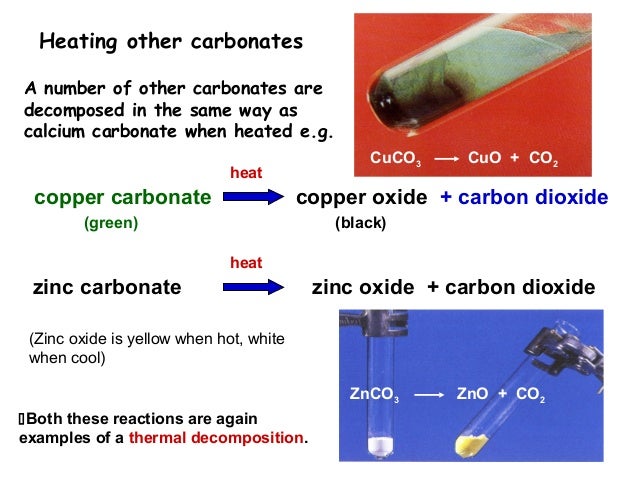
Copper Carbonate (CuCO3) decomposes by heat to form either one of two oxides, namely Copper (I) Oxide (Cu2O) and Copper(II) Oxide (CuO). ... Column II carbonates are decomposed by heat to form corresponding oxides and carbon dioxide.
The reaction is call a Thermal Decomposition. These reactions always need heat.
Decomposition reactions are exactly as you would expect: a breakdown or separation of a chemical compound into elements or more simple compounds. During the process, substance AB decomposes or breaks apart to produce substance A and B.
AB → A + B

Decomposition of Copper Carbonate - Video
Equipment without collectig tube
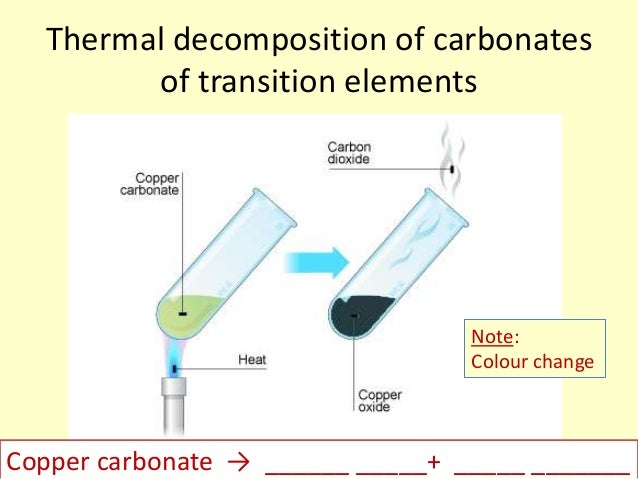
With Collecting Tube

Observations
XXXXXXX
Word Equation
Metal Carbonate + Heat = Metal Oxide + CO2
Copper Carbonate + Heat = Copper Oxide + Carbon Dioxide
Chemical Equation
CUCO3 (s) = CUO (s) + CO2 (g)
Balanced Full Equation
As above
Further Research
Wiki of Chemistry
This type of reaction is called thermal decomposition.
Thermal decomposition is an example of an endothermic reaction, a reaction that gains energy from the surroundings.
Excellence links
https://en.wikipedia.org/wiki/Chemical_decomposition
https://www.bbc.com/bitesize/guides/zqd2mp3/revision/5
No comments:
Post a Comment