Part 1
Recap Combination & Decombination (5mins)
Test you knowledge (Internal Practice) (15mins)
Part 2
Recap Displacement (5mins)
Test you knowledge (Excellence Internal Practice - will be marked ) (20 mins)
Friday
Part 3
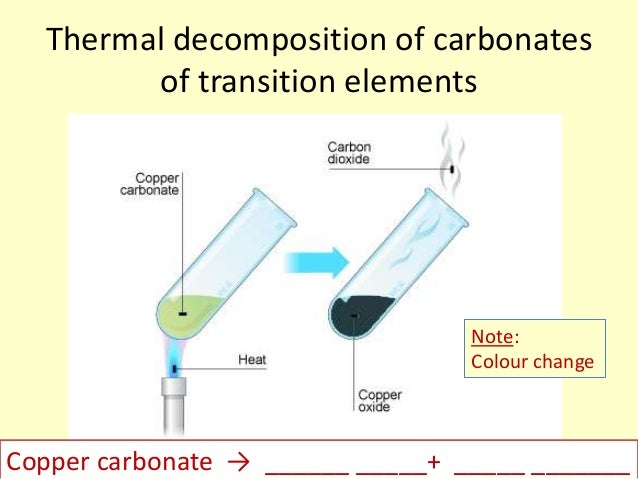
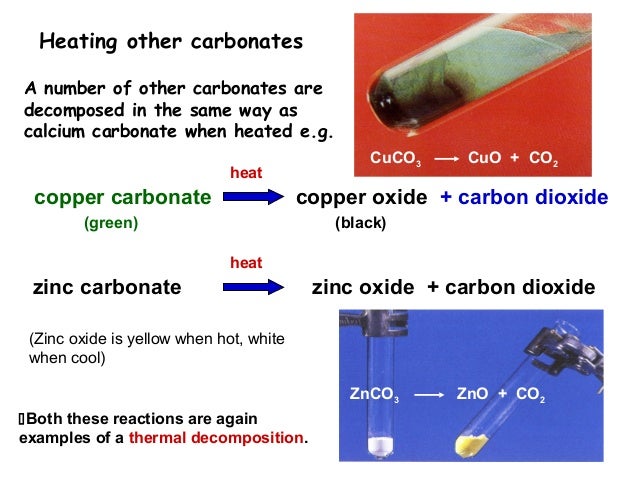
Exchange Reaction Recap
Test Your Knowledge
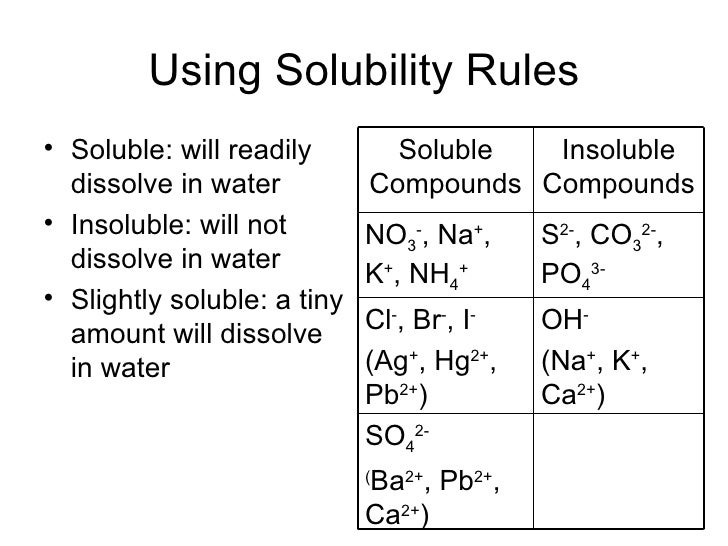
Solubility Table
1.
All nitrates are soluble.
2.
All sodium and potassium compounds
are soluble.
3.
All chlorides are soluble except silver chloride and lead
chloride.
4.
All sulfates are soluble except barium sulfate, lead sulfate, calcium
sulfate.
5.
All carbonates are insoluble except sodium and potassium carbonates.
6.
All hydroxides are insoluble except sodium and potassium hydroxides.
|
Reactivity Series of Metals
1.
Potassium
2.
Sodium
3.
Lithium
4.
Calcium
5.
Magnesium
6.
Aluminium
7.
Zinc
8.
Iron
9.
Tin
10.
Lead
11.
Copper
12.
Silver
13.
Gold
14.
Platinum
How to write an answer. (5 steps to Merit & Excellence)
RULE :A displacement reaction in where a more reactive metal displaces a less reactive metal from a solution.
OBSERVATION: When the Magnesium metal was placed in the Copper Sulfate solution, we saw Copper crystals form as the Magnesium was corroded.
WHY/JUSTIFY
This was because Magnesium is a more reactive metal than Copper and it was reacting with the Sulfate in the solution.
This was because Magnesium is a more reactive metal than Copper and it was reacting with the Sulfate in the solution.
As the symbol equation shows as well as lead, a solution of zinc nitrate was being formed also.
WORD EQUATION
WORD EQUATION
SYMBOL EQUATION
Zn(s) + Pb(NO3)(aq) → Pb(s) + Zn(NO3)2(aq)
Zn(s) + Pb(NO3)(aq) → Pb(s) + Zn(NO3)2(aq)